Standardize Customize Optimize
From the grand overview to the granular detail, securely manage your department, metrics and QA data, anytime, anywhere.
QA Management
- Perform QA, integrate results and manage devices from one standard platform
Dashboards
- Each area of the software starts with a filterable dashboard overview, personalized to the individual user’s role and responsibilities. Physicists can easily track the status of scheduled QA, machine downtime, equipment-related tasks and more.
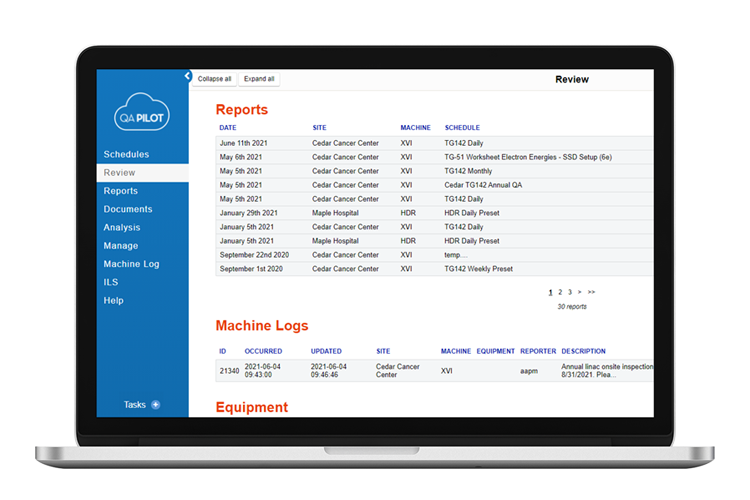
Periodic QA
- Daily, monthly, and annual QA are made easy with preset tests and templates for all QA tests outlined in TG-142. Configure these and custom tests into the appropriate standardized workflow for your department. Data from a wide variety sources can be uploaded effortlessly to QA templates and automatically included in QA reports.
|
 |
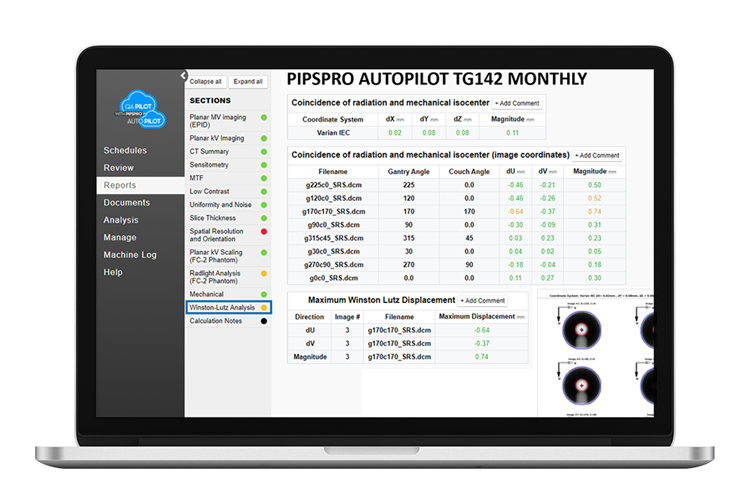 |
Automated Image Analysis with PIPSpro™ AutoPilot
- Upload images to PIPSpro AutoPilot schedules for automatic image analysis and reporting, eliminating tedious manual intervention.
Integrated Solution
- The addition of PIPSpro AutoPilot to the QA Pilot platform eliminates the need to access external software programs and data to complete TG142 monthly (and annual) QA schedules. Quick DICOM file uploads to assigned QA templates are all that’s needed for automatic analysis and reporting of image-based tests within this cloud-based application.
Vendor Neutral
- Automated image analysis tests for IGRT, MLC QA and VMAT QA using the gold standard PIPSpro Phantom Set, linac manufacturer-provided phantoms and other common imaging phantoms are included.
|
Administrative Management
- Streamline operations and improve communication throughout the radiation therapy department
Reports and documents
- Use QA Pilot’s reports and documents repositories to organize departmental metrics for better administrative and regulatory oversight. The valuable information contained in reports and documents is foundational to implementing standardization goals across sites and machines within your organization.
Communication and tasks
- Administrative communication can be pushed out to sites, user types and/or individuals with a required acknowledgment. Machine and device service issues are easily communicated to vendors and personnel via the HIPPA-compliant email service built into the software. Tasks are assigned with due dates from anywhere in the software.
|
 |
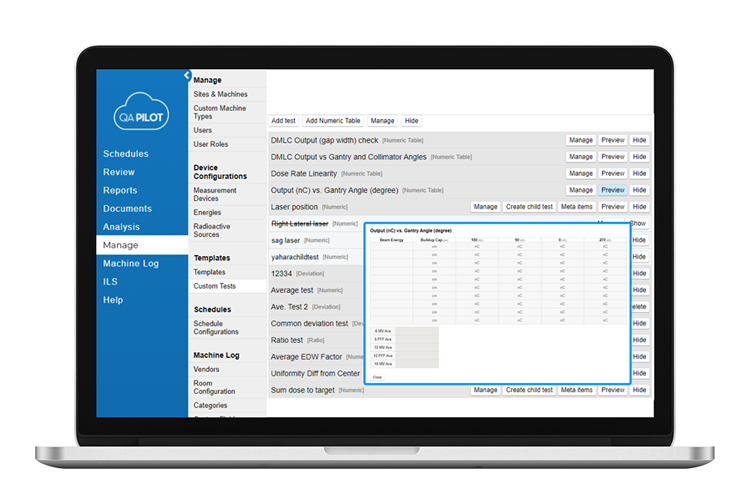 |
Customize tests, templates and workflows
- Whether for a single site, or a large multi-site institution, customization is key to implementing a comprehensive QA management system.
It’s your workflow
- Preconfigured tests and templates are just the starting point as they can be configured according to your departmental workflow. Create a custom test once and apply it to any template. Once a template has been created, schedule it to any machine within the organization and further customized if necessary for specific linacs.
It’s your data
- Replacing spreadsheets is easier than ever with the variety of mathematical functions that can be performed on data/metrics entered in QA schedules. Averages, ratios, deviations, common denominators and averaged numerators are just some of the functions available. Data tables allow multiple functions to be carried out on entries by row, column, or selected cells.
|
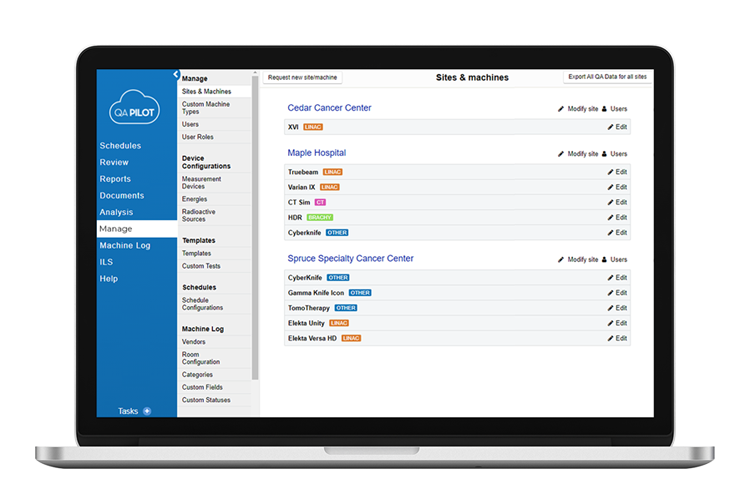
Customize management oversight
- Whether for a single site, or a large multi-site institution, customization is key to implementing a successful management oversight program.
It’s your institution
- Extensive customization features in the software mean that your institution is accurately characterized for performance oversight. Everything from sites to users to processes to workflows to assignments and scheduling are customizable.
It’s your efficiency
- The key to making efficiency gains is understanding the what, when, how and why problems happen so that patients are treated safely in a timely manner. Machine logs are created to track equipment downtime for linacs and other equipment. The ILS, Incident Learning System, is used to record (potentially) adverse events so that they can then be assessed and corrective action taken. All incidents logged in the ILS portion of the software are encrypted upon entry and only viewable by those assigned to the ILS committee.
|
 |
Optimize Results
Enjoy optimal flexibility, scalability and affordability to manage the wealth of data/metrics produced by your organization.
|
| |
|
|
|
Interoperability
Vendor-neutral software means allowing automatic data integration from a variety of sources.
|
Process Improvement
Use metrics collected for machines, devices and QA activities to drive process improvement within a department or throughout the entire organization.
|
Predictive Analytics
Apply statistical techniques to trended data across sites and machines to identify potential issues and take action before it impacts continuity of care.
|





